The innovations that could bring us closer to curing diabetes
With 5 per cent of the world’s population receiving a diabetes treatment that costs trillions of pounds a year, think how much people would be willing to pay for a cure, writes Steven Cutts

Diabetes is a disease of our times. More than 400 million people are currently living with diabetes – about 5 per cent of the global population. These patients can be divided into two groups, those that require insulin and those that don’t.
The economic cost of coping with this condition is immense. Europe alone spends around €90bn a year just to keep its diabetic population alive. Most, if not all, insulin-dependent diabetic patients would die very quickly if they couldn’t get access to their insulin and since the hormone was first mass-produced for human use, there has been little if any progress in the way that diabetic patients are treated. In the first few decades after its introduction, millions of lives were saved but nobody was seriously talking about a cure. Virtually all diabetic doctors focus on palliation.
When we look at different animals around the world it turns out that pretty much all of them require insulin and what’s striking about the structure of the insulin molecule is its consistency. It has been preserved throughout evolution, so much so that fish insulin will produce a discernible response if we inject it into humans. It doesn’t work particularly well – and I wouldn’t want to be treated by it myself – but man and fish haven’t shared a common ancestor for more than a hundred million years so the fact that it works at all is more than astonishing.
In type 1 diabetes, the body stops producing insulin and the patient loses control of their blood sugar level. If this continues for any length of time, the patient will die. It is treated with insulin injections that usually have to be given every day. Since the 1920s, it has been possible to extract insulin from the pancreas of pigs and cows. Both of these animals are routinely slaughtered in large numbers. In an evolutionary sense, both species are quite close to man.
And that’s about it. We know how to treat it and we know how to keep people with the condition alive but we’re no closer to finding a cure today. But if a cure could be found, the implications would be fantastic. Entire clinics would evaporate overnight. It would do for health service spending what the end of the Cold War did to the arms race. The rewards on offer to whichever genius comes up with a solution defy the imagination and the people who are backing various start-up companies in this field are more than aware of this.
Several lines of attack are now being considered.
In type 1 diabetes, the body’s own immune system attacks special cells in the pancreas that produce insulin. These cells are known as beta cells and they cluster with other cells to form islets.
Why does the body pick on the critically important islet cells in this way? We don’t know. However, it has long been understood that there is a seasonal variation in the onset of new cases and for this reason – if no other – many doctors believe that diabetes may have a viral origin. If there is any truth in this theory, surely we ought to be able to identify the virus? And if we could identify it, then we ought to be able to devise a vaccine that would enable us to eliminate the disease in much the same way that we previously saw off the really devastating condition polio?

The process of self-destruction takes many months and in some cases, doctors become aware of the illness before the insulin-producing cells have been completely destroyed. Some doctors believe that at least 10 per cent of those cells might still be active and even if we saved this modest amount of good tissue, it might enable the patient to use far fewer injections of insulin.
At the time of writing, the French are working on a vaccine for diabetes that would downgrade the body’s enthusiasm to attack its own islet cells and help preserve what little insulin-producing tissue still exists.
Given that this kind of work is still at an experimental stage, it seems reasonable to explore other options too. We know that insulin is made in special islet cells that are only found in the pancreas. Surely we could cure diabetes by offering to transplant an entire pancreas from one person to another.
But if a cure could be found, the implications would be fantastic. Entire clinics would evaporate overnight. It would do for health service spending what the end of the Cold War did to the arms race
In fact, there has been some success in transplanting a single kidney and a pancreas in one procedure. Quite a lot of end-stage diabetics have gone into kidney failure and by doing a double transplant we can re-establish kidney function and insulin production in one procedure. Needless to say, transplant surgery is expensive and requires the patient to take anti-rejection drugs for the rest of their life. Transplant surgery also suffers from a perpetual shortage of donors and our increasingly safe home and working environment is only making this situation worse.
Many years ago, people began to speculate that it might be possible to inject small clusters of islet cells into a diabetic patient. If these cells could then survive inside the recipient they might be persuaded to produce insulin again, thus freeing the patient from the need to inject themselves with insulin.
This procedure has been around for some time. It can even be done as a day case under local anaesthetic. The doctor injects the islet cells into the vein that runs into the liver. The injected cells soon impact the liver and (hopefully) begin to flourish in this new environment. In time, they even produce insulin and the patient’s symptoms begin to fade.
However, this is still transplant surgery albeit at a microscopic level and the patient needs to take immunosuppressive drugs to stop their own immune system rejecting all of the foreign islet cells that have just been injected. Even when this procedure is successful, it’s unusual for the patient to be totally free of insulin injections. In addition, the effects are temporary. Within a year or two, the situation may deteriorate and the patient has to take insulin again, although there’s quite a lot of evidence that the actual dose required does fall, meaning fewer encounters with a rather unpleasant needle.
Researchers are also looking at how to alter or shield a group of islet cells before we inject them into the recipient. In this way, they might avoid being rejected by the patient’s immune system. Even when this procedure is successful, it’s unusual for the patient to not require any insulin injections at all. Within a year or two, the situation deteriorates and the patient has to take insulin all over again although there’s some evidence that the actual dose required does fall, meaning fewer sessions with an unpleasant needle.
Some insulin-dependent diabetic patients experience hypos when their blood sugar falls to perilously low levels. Some of these patients then black out without warning. Most diabetic patients experience a strange subjective sense of altered consciousness as their blood sugar falls. In time, they learn how to recognise an imminent hypo and know when to swallow some emergency sugar. However, some patients don’t experience the warning signs and this is a particularly dangerous time for a diabetic, especially those who live alone.
400m
people live with diabetes worldwide
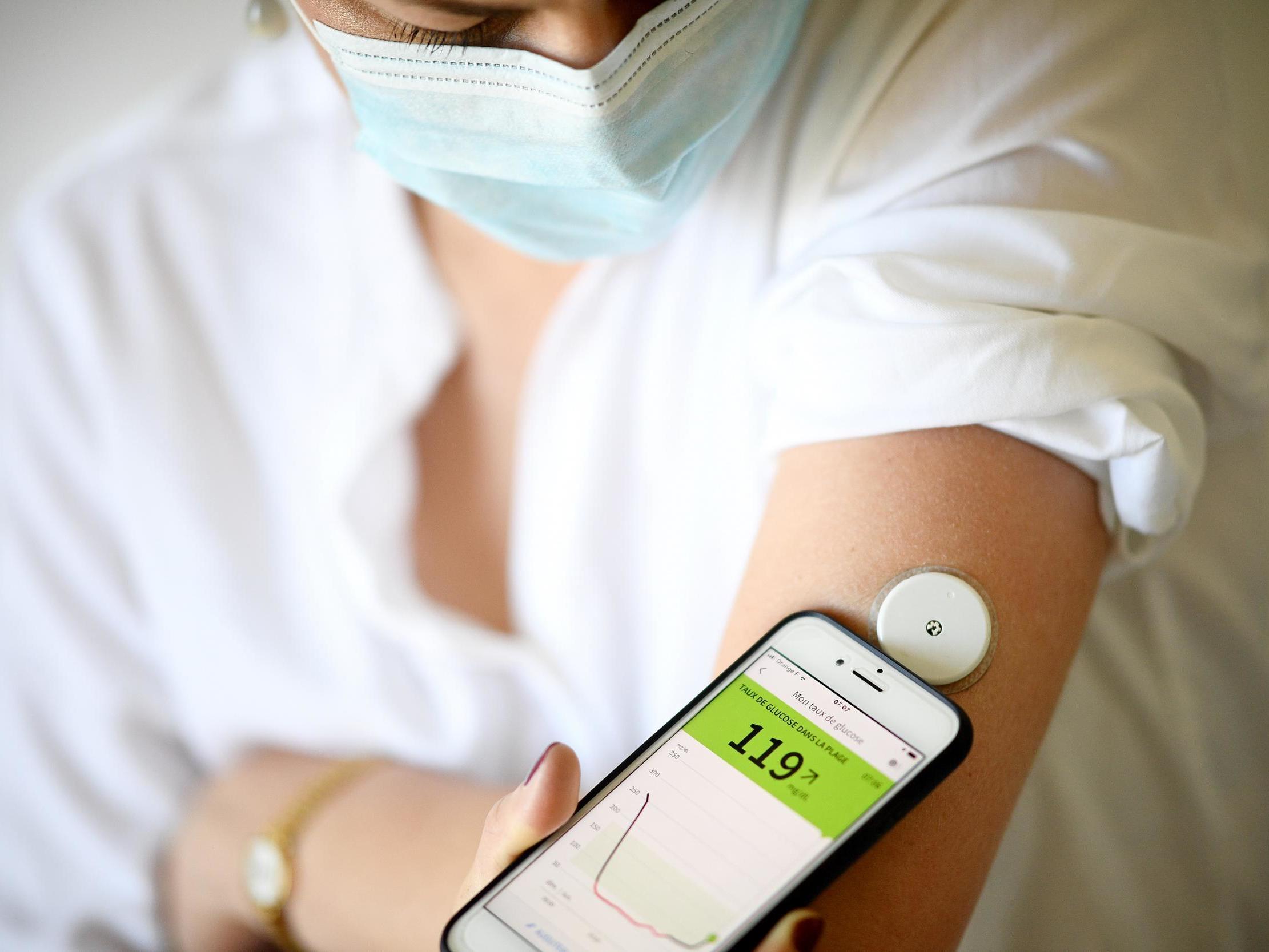
Diabetic patients have to learn how to measure their own blood sugar by placing a droplet of blood on a test device. Most of the time, diabetic patients look pretty normal and live among us unnoticed, but in private some of their daily rituals are a real nuisance. If we could eliminate the need for patients to repeatedly prick themselves in pursuit of a small blood sample, their lives would be significantly improved.
In one of the more colourful sidelines of the American tech company, Google commissioned two groups to design and build a contact lens that might monitor a person’s blood sugar level without the need for regular blood samples. The project began in 2014 and was abandoned in 2018 when the researchers acknowledged that the amount of sugar in a teardrop isn’t always the same as that seen in their blood. Research is like this, but there are so many groups trying to make a breakthrough that it’s hard to imagine that none of them will ever succeed.
Later this year, the Americans will probably classify islet cell transplants as a legitimate treatment for diabetes. This particular decision might sound a bit odd, given that they’ve been using islet cell transplantation for the past 20 years or so. However, so far it has been regarded as an experimental treatment and only a small number of people have ever been treated. Part of the problem with islet cell transplant lies in the supply of oxygen to the islet cells. About 60 per cent of the islet cells die from lack of oxygen before they can establish a blood supply in the liver. Some doctors believe that the side effects of the immunosuppressive medication are so unpleasant that the patients would be better served by continuing to take insulin.
Researchers have long had an interest in trying to persuade other tissues in the patient’s own body to transform into beta cells. In effect, the patient would renew their ability to make insulin. One advantage of this approach is that if it worked, the cells making insulin would belong to the actual patient and wouldn’t be regarded as alien by the body’s immune system.
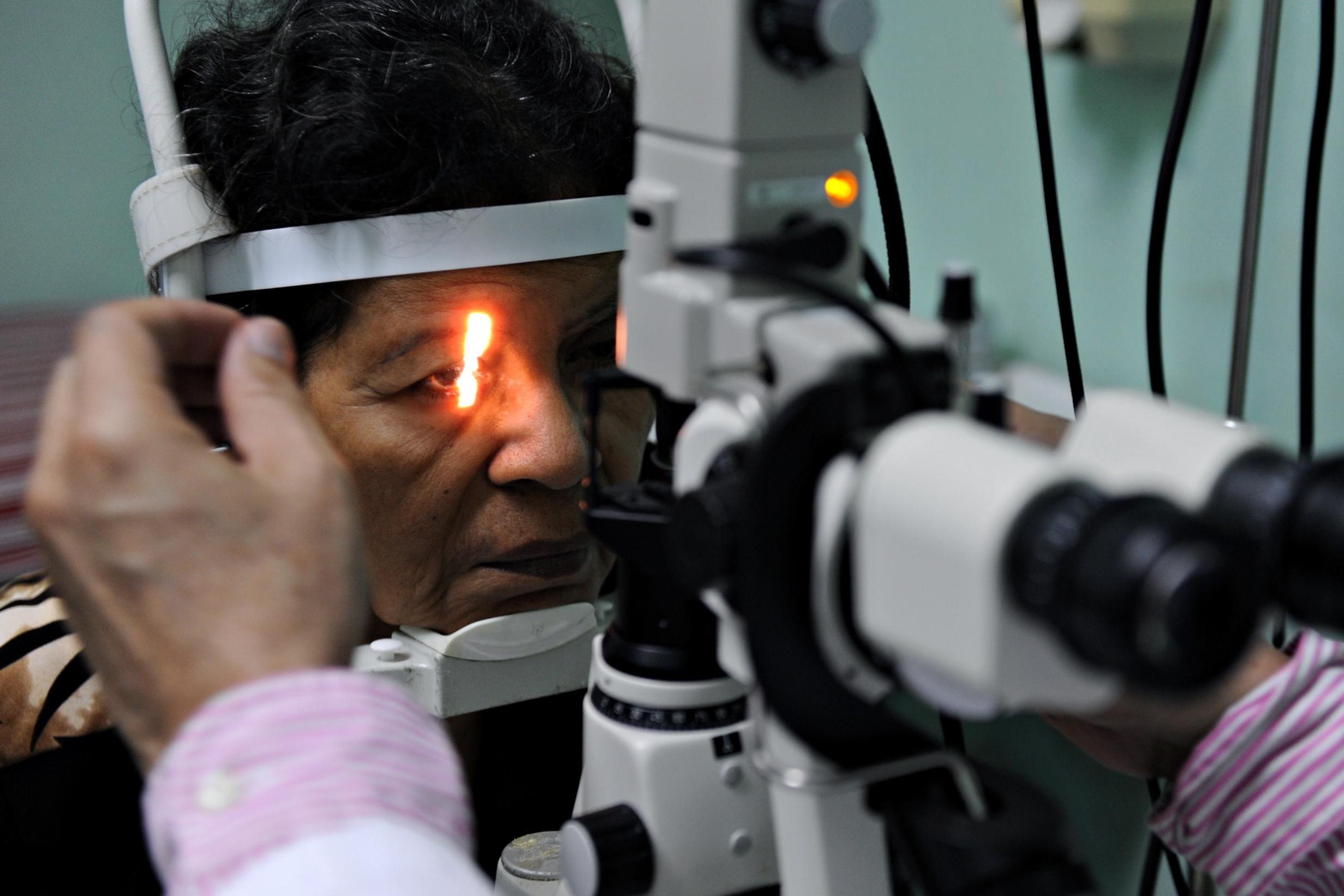
In 2019, researchers at the Washington University School of Medicine in St Louis published evidence that it might be possible to manufacture insulin-producing tissue from human stem cells. Once upon a time each and every person on this planet was one cell. That one fertilised egg divided repeatedly over the course of nine months and eventually produced a complex and functional human being containing all kinds of specialist cells. One of these cell types was, of course, islet cells that live in your pancreas. So, the idea that one cell might be transformed into any other cell is credible, but in practice we’re only just learning how to make it happen. The St Louis study managed to persuade stem cells to look like beta cells and actually make insulin in mice.
And at this point, I’d just like to explain a note of caution: the history of medical research is awash with people who can make something work in a mouse or some other experimental situation. Making the same thing work in a human being isn’t so easy and more than one scientist has been left with egg on their face by claiming that it is.
Scientists at the University of Pittsburgh managed to deliver gene therapy to the alpha cells that are also found in pancreas. Alpha cells extracted from animals were successfully turned into beta cells. Remember that beta cells are the ones that actually produce insulin. Later on, they seemed to achieve the same result in a living mouse and claimed that blood glucose was brought under control for as long as four months. As with islet cell transplants, this transformation was far from permanent.
The Diabetes Research Centres in the United States is also working on a technique that could be injected into the patient as a transplant but shielded from the patient’s immune system by packaging the cells in a special barrier. A small number of people have had this treatment already and initial results are said to be promising. Using this system, the donated cells are wrapped in a bio-engineered scaffold and inserted into a layer of fatty tissue in the abdomen known as the omentum. The group involved claim that in the future this technique might enable us to transplant islet cells without the need for immunosuppressive drugs. If this is true and if the transplanted cells removed the need for insulin injections then they could put in a claim for having effectively cured diabetes.
In the UK, Islexa is attempting to persuade surviving cells in the patient’s own pancreas to start producing insulin. Islexa uses a viral vector (ie the genetic instructions to the cell are incorporated into a man-made virus) and the cell is then infected with the virus. With luck, the infected cell starts to produce insulin. In effect, we will have metamorphosed ordinary pancreatic cells back into the missing beta cells that the diabetes disease process originally destroyed. This approach would enable us to avoid using immunosuppressive drugs. Better yet the new, insulin-producing tissue would be in exactly the same place it is meant to be: in the patient’s own pancreas.
Over in Belgium, another high-tech startup, Orgenesis, is using a similar approach to persuade the patient’s liver cells to start producing insulin. In case you hadn’t spotted it already, I probably ought to point out that the competition in this field is absolutely cutthroat. Whichever country manages to pull this one off will upgrade their national GDP for the next 20 years.
If we’re willing to pay €90bn a year to palliate diabetes in Europe, think how much people will be willing to pay for a cure.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments