Indonesia becomes latest country to raise alarm over syrup medicines after 99 children die
There has been a jump in kidney disease cases in the country since August
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
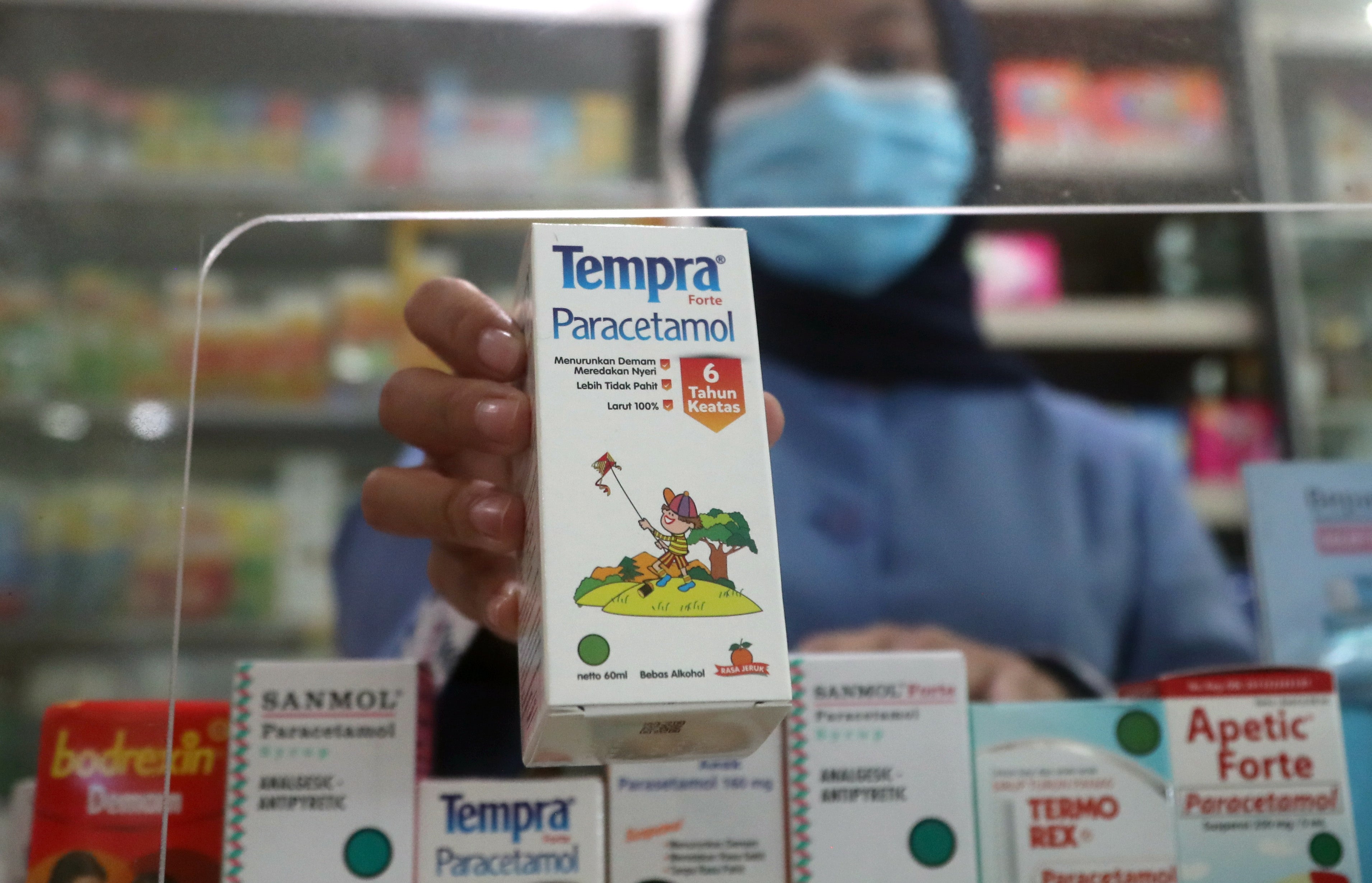
Your support makes all the difference.Indonesia has become the latest country to ban sales of all syrup and liquid medication linked to the deaths of nearly 100 children.
The move comes after The Gambia sounded the alarm over a cough syrup manufacturer in India, the products of which have been linked to the deaths of at least 70 children.
Indonesia’s health minister said some syrup medicine had ethylene glycol and diethylene glycol, ingredients which caused acute kidney injuries (AKI) in 99 young children this year, leading to their deaths.
Authorities reported around 200 cases of AKI in children, most of who were under the age of five.
Indonesia’s food and drug agency said the particular products identified in The Gambia were not available locally.
“Some syrups that were used by AKI child patients under five were proven to contain ethylene glycol and diethylene glycol that were not supposed to be there, or of very little amount,” said Budi Gunadi Sadikin.
Mr Budi said the real death toll could be greater than 99 fatalities. However, he did not say in how many AKI patients the harmful ingredient was detected, citing an ongoing investigation.
“As a precaution, the ministry has asked all health workers in health facilities not to prescribe liquid medicine or syrup temporarily... we also asked drug stores to temporarily stop non-prescription liquid medicine or syrup sales until the investigation is completed,” health ministry spokesperson Muhammad Syahril Mansyur said on Thursday.
The number of AKI cases in the country began increasing in January this year and continued to rise in late August, Mr Mansyur said. He added that the probe was launched last week.
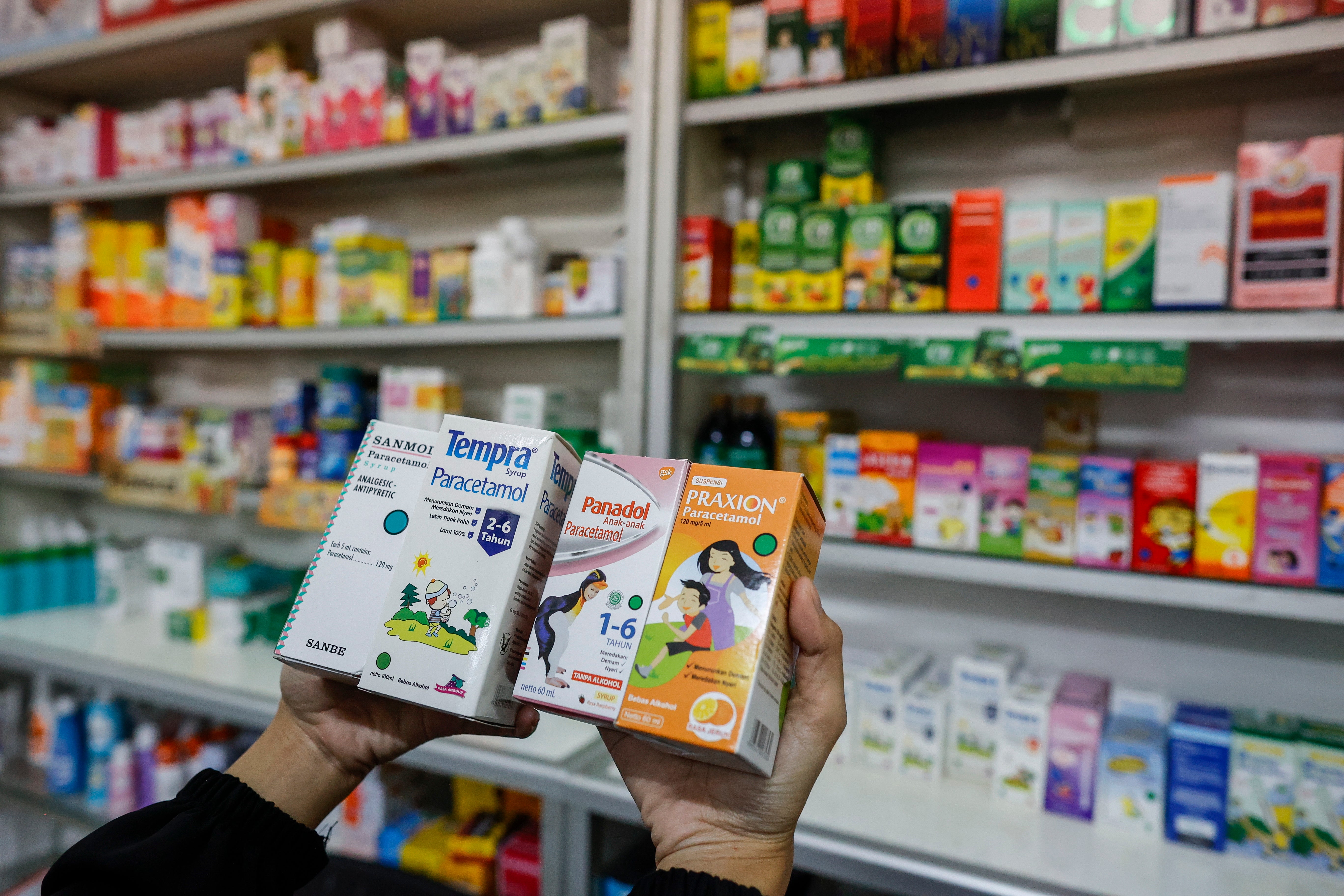
The country has formed a team consisting of local health and paediatrics officials and WHO representatives to investigate the faulty medicines.
“Since late August 2022, the ministry and the paediatrician association have received increasing reports of acute kidney injury. The jump is sharp,” he said.
He added that 65 per cent of cases had been treated in the capital Jakarta.
Health authorities have not yet disclosed the brands or the type of syrups linked to the sickness.
The World Health Organisation (WHO) issued a global warning over four cough syrups that were linked to the deaths of 70 children in The Gambia. The health agency said these products were made in India and imported via a US-based company.
It said the syrups contained “unacceptable amounts” of diethylene glycol and ethylene glycol and that the substances are “potentially linked with acute kidney injuries”, in a major setback to India, which produces a third of the world’s medicines.
The cough syrups being investigated by India are Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and Magrip N Cold Syrup.
All batches of the four products will be marked unsafe by the WHO until they can be analysed by federal regulatory authorities, ordered the UN’s health agency.






Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments