WHO flags India-made ‘contaminated’ cough syrup with ‘unacceptable amounts’ of toxic chemicals
‘Substandard’ medicine is latest in series of drugs manufactured in India said to be of poor quality
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.A batch of a “contaminated” cough syrup made in India and found in the Marshall Islands and Micronesia has been flagged by the World Health Organisation (WHO).
This marks the latest in a series of medicines manufactured by India-based pharmaceutical firms and distributed in different countries that are alleged to be of poor quality and harmful to use.
In countries like The Gambia and Uzbekistan, consumption of syrups manufactured in India have been linked to the deaths of several children.
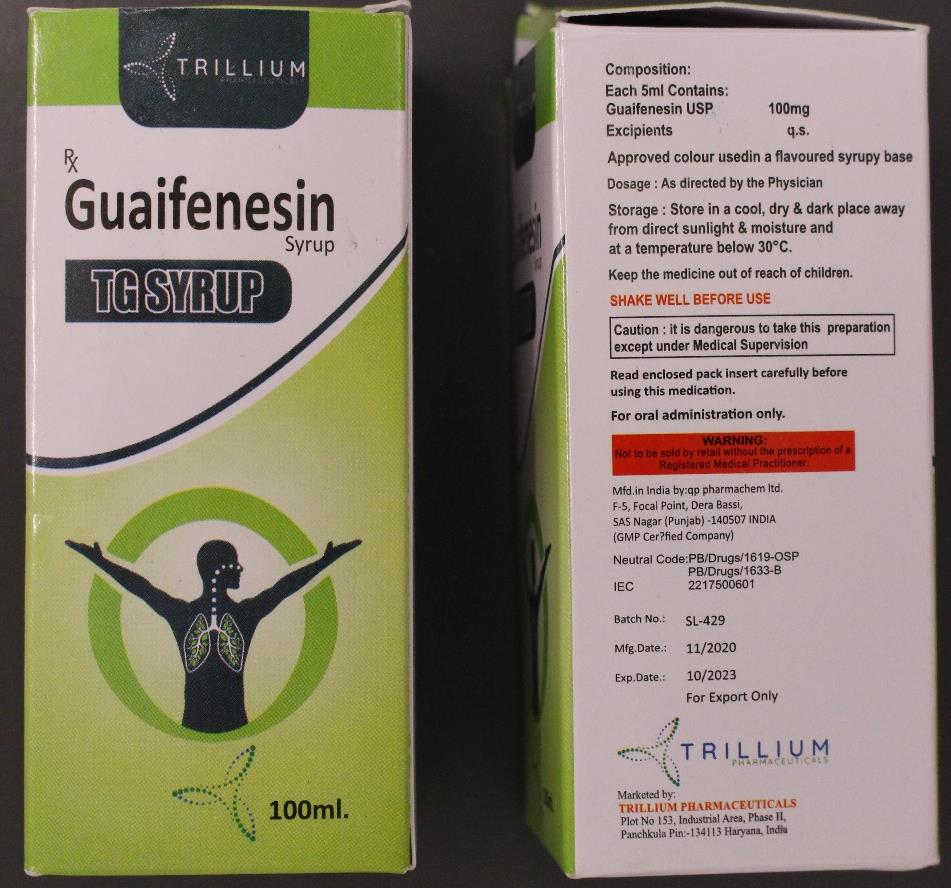
The “substandard” medicine flagged by the WHO in a statement on Tuesday is known as Guaifenesin Syrup TG and is used to relieve chest congestion and the symptoms of cough.
The drug was brought to the WHO’s attention earlier in April.
The medicine was manufactured by QP Pharmachem Ltd, based out of the northern Punjab state and marketed by Trillium Pharma in neighbouring Haryana.
Neither of the firms “have provided guarantees to WHO on the safety and quality of these products”, it said.
The world health agency, however, did not say if any deaths or injuries were linked to Guaifenesin.
WHO said there were “unacceptable amounts” of diethylene glycol and ethylene glycol found in Guaifenesin. The toxic chemicals have been found in cough syrups in other countries as well.
The chemicals were found in large quantities in four syrups sold in Gambia. The deaths of 69 children late last year were linked to the use of the syrups manufactured in India.
In Uzbekistan, the deaths of at least 19 children last year were linked to one India-manufactured cough syrup. The syrup, Doc-1 Max, was found by Uzbekistan’s health ministry to have contained ethylene glycol.
The Indian manufacturer of Doc-1 Max was ordered to halt production of the syrup in the wake of the deaths.
In the wake of the deaths reported from The Gambia in October last year, Indonesia halted sales of all syrup and liquid medication after they were linked to the deaths of hundreds of children there.
Indonesia’s health minister said some of the medicines had both the chemicals present.
Diethylene glycol and ethylene glycol are said to cause acute kidney injuries and can prove fatal.
The former is found in antifreeze and e-cigarettes and is said to make cheap wine taste richer as well. It can also be used as an engine coolant.
The managing director of QP Pharmachem Ltd, that manufactures Guaifenesin, told the BBC that the drug was not intended for the Marshall Islands and Micronesia.
Sudhir Pathak said his firm had exported a batch of 18,346 bottles to Cambodia after getting regulatory approval.
“We did not send these bottles to the Pacific region, and they were not certified for use there. We don’t know under what circumstances and conditions these bottles reached the Marshall Islands and Micronesia,” he said.
Mr Pathak said a legal notice had been sent to the firm that exported the medicines to Cambodia.
The BBC said marketer Trillium Pharma was unavailable for comment.
India’s government has not reacted to the WHO’s warning on Guaifenesin Syrup TG yet.
Earlier this month, the US Food and Drug Administration (FDA) had highlighted serious lapses in the manufacture of eye drops linked to three deaths.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments