First licence to genetically modify human embryos could revolutionise IVF treatments, scientists say
However, some scientists believe the research could lead to calls for the creation of GM babies
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
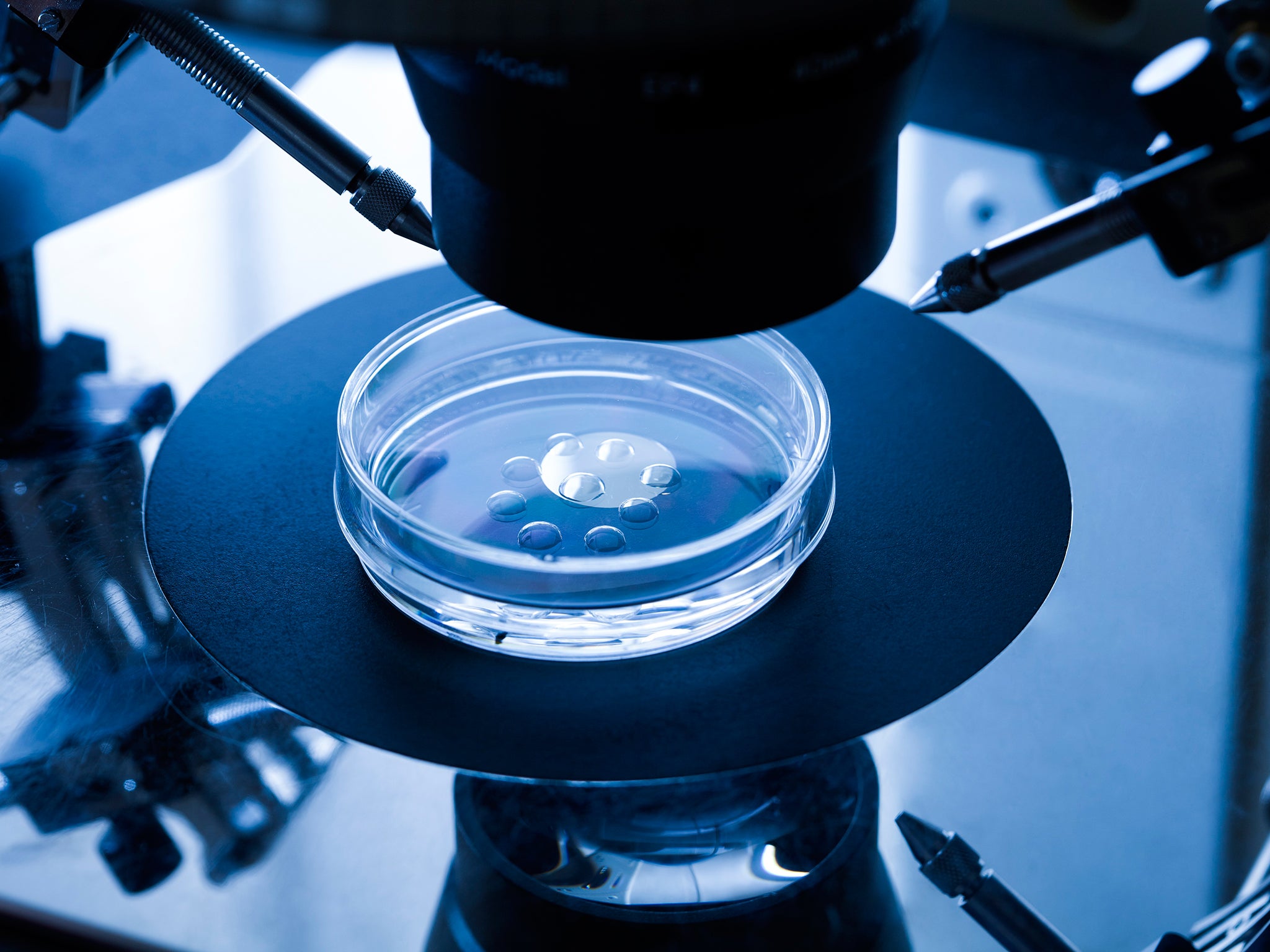
Your support makes all the difference.The first licence in Britain to “edit” the genes of human IVF embryos should lead to a better understanding of the fundamental fertility problems suffered by thousands of couples trying to start a family, scientists have said.
Approving the licence application by the Crick Institute in London would provide vital details of how genes work in human embryos less than six-days old, which could ultimately help to improve the chances of a successful pregnancy in women undergoing IVF treatment, the researchers said.
Scientists advising the Human Fertilisation and Embryology Authority (HFEA) also said that this knowledge could not come about without experiments on spare human IVF embryos donated for research. The embryos of laboratory mice have proven to be too different to shed much further light on the arcane genetics of early human development, they said.
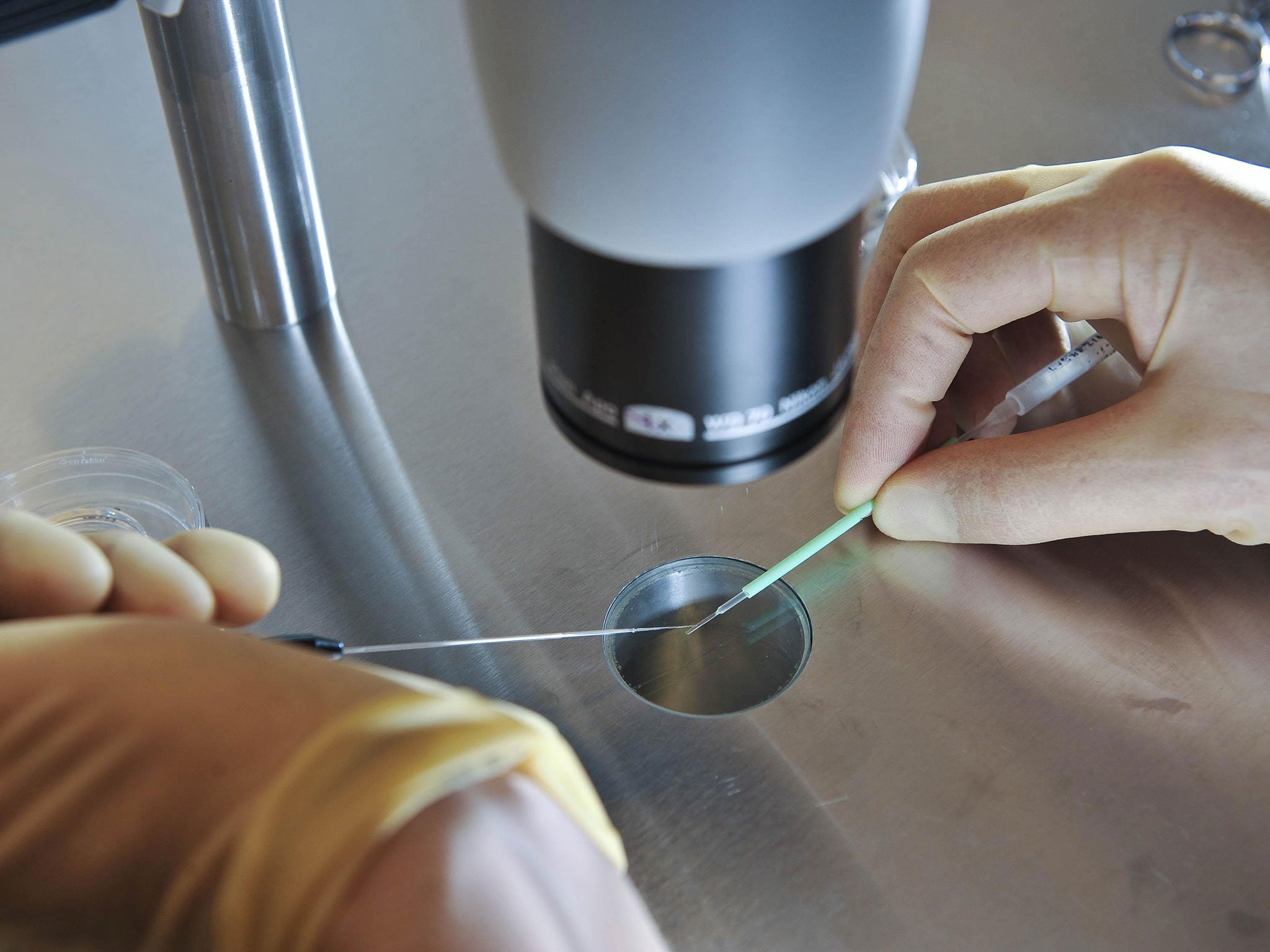
The HFEA announced that it has approved the extension of an existing research licence held by the Crick Institute that would for the first time in Britain allow the use of a gene-editing technique called Crispr-Cas9 to remove small segments of DNA in order to disable key genes suspected of being involved in the development of early human embryos.
About 30 or 40 genetically-modified embryos are expected to be used in the study but none will be allowed to survive beyond five or six days – well within the legal limit of 14 days – and it will remain illegal for researchers to attempt to implant them into the womb.
However, some scientists believe the research could lead to calls for the creation of GM babies if “germline” genetic modification can be shown to have a proven medical benefit, such as the treatment of infertility.
“We will also learn about the use of the techniques in human embryos, how efficient and accurate they are, which will help inform the debate about whether germline treatments to make heritable changes would be practical and safe,” said Professor Robin Lovell-Badge, an expert in mammalian reproduction at the Crick Institute.
The minutes of the HFEA’s scientific committee, which approved the licence application last week, were released on 1 February. They state that progress in this field using mouse embryos alone is hampered by the fact that they have proven to be too different to human embryos.
“The committee found that the use of human embryos is necessary because the proposed research aims to study the role of gene products in human embryos that are not present in mouse embryos at the same stage,” the Minutes say.
“Moreover, human embryonic development is significantly different to that of animal model species in a number of respects and, additionally, not all functions required for preimplantation development can be modelled in embryonic stem cells,” they add.
Kathy Niakin, who will lead the project at the Crick Institute, said that blocking the function of a set of key keys using Crispr-Cas9 in one-day-old embryos would help to understand why the IVF embryos of some women repeatedly fail to develop when transplanted in the womb.
Although her HFEA licence application has now been approved, the Crick Institute still has to be given approval by the Cambridge Central Research Ethics Committee before the study can proceed, which is likely to be later this year.
Professor Lovell-Badge said that Dr Niakin’s study with Crispr-Cas9 will address the role of specific genes in determining the development of the early human embryo in the first few days after fertilisation.
“The assumption had been that what is true for the mouse will also be true for humans, but we now know through the work carried out by Kathy and others over the last few years that this is unlikely to be true; indeed there seem to be many differences,” Professor Lovell-Badge said.
“The approval of her licence gives the exciting prospect that we will at last begin to understand how the different cell types are specified at these pre-implantation stages in the human embryo,” he said.
“This fundamental knowledge may also have practical benefits in helping to improve success rates for IVF and for both the establishment and maintenance of pregnancy - in ways that will not depend on genome-editing methods,” he added.
Q&A: ‘editing’ human embryos
Q | What has been announced?
A | The Human Fertilisation and Embryology Authority (HFEA), which regulates fertility clinics and research, has granted a team of scientists investigating the causes of miscarriage permission to “edit” the genes of human embryos.
Q | Why is this move so controversial?
A | Until now, scientists in the UK have not been allowed to manipulate inherited “germ line” DNA in eggs, sperm and embryos. The new licence granted to scientists at the Francis Crick Institute in London marks a significant turning point. Critics say it is the start of a “slippery slope” that could eventually lead to the creation of “designer babies”.
Q | What will the work entail?
A | The team led by Dr Kathy Niakan will use a powerful new gene-editing technique called Crispr-Cas9 to make precise changes to the DNA in early-stage embryos.
Q | Why is the research being carried out?
A | It is part of a far-reaching investigation into what happens to an embryo in the first seven days after fertilisation, and the causes of miscarriage, which remain largely unknown.
Q | Will the changed embryos develop into babies?
A | No, there are strict boundaries to the research. The embryos, donated by couples undergoing IVF treatment who no longer need them, cannot under any circumstances be implanted into a womb. They can only be used for basic scientific research and legally must be destroyed after two weeks.
Q | Is it the first time in the world that this has been allowed?
A | No. Last year a group of Chinese scientists became the first to announce that they had genetically manipulated human IVF embryos for research.
Q | What is Crispr-Cas9?
A | An incredibly powerful new technology that makes it possible to “cut and paste” DNA with high precision. Using the system scientists can target specific sections of DNA, delete them, and if necessary, insert new genetic sequences.
Q | How does Crispr-Cas9 work?
A | In its most basic form, the Crispr-Cas9 “tool kit” consists of a small piece of RNA – a genetic molecule closely related to DNA – and an enzyme protein called Cas9. The RNA component is programmed to latch on to a specific DNA sequence. Then Cas9 slices through the strands of DNA, like a pair of molecular scissors.
When DNA is cut in cells, repair systems kick in to try to fix the damage. This is exploited in more advanced Crispr systems which include additional DNA the cell can use to mend the break, thereby making it possible to rewrite the genetic code.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments