Exclusive: 'Jaw-dropping' breakthrough hailed as landmark in fight against hereditary diseases as Crispr technique heralds genetic revolution
Development to revolutionise study and treatment of a range of diseases from cancer, incurable viruses such as HIV to inherited genetic disorders such as sickle-cell anaemia and Huntington’s disease
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.A breakthrough in genetics – described as “jaw-dropping” by one Nobel scientist – has created intense excitement among DNA experts around the world who believe the discovery will transform their ability to edit the genomes of all living organisms, including humans.
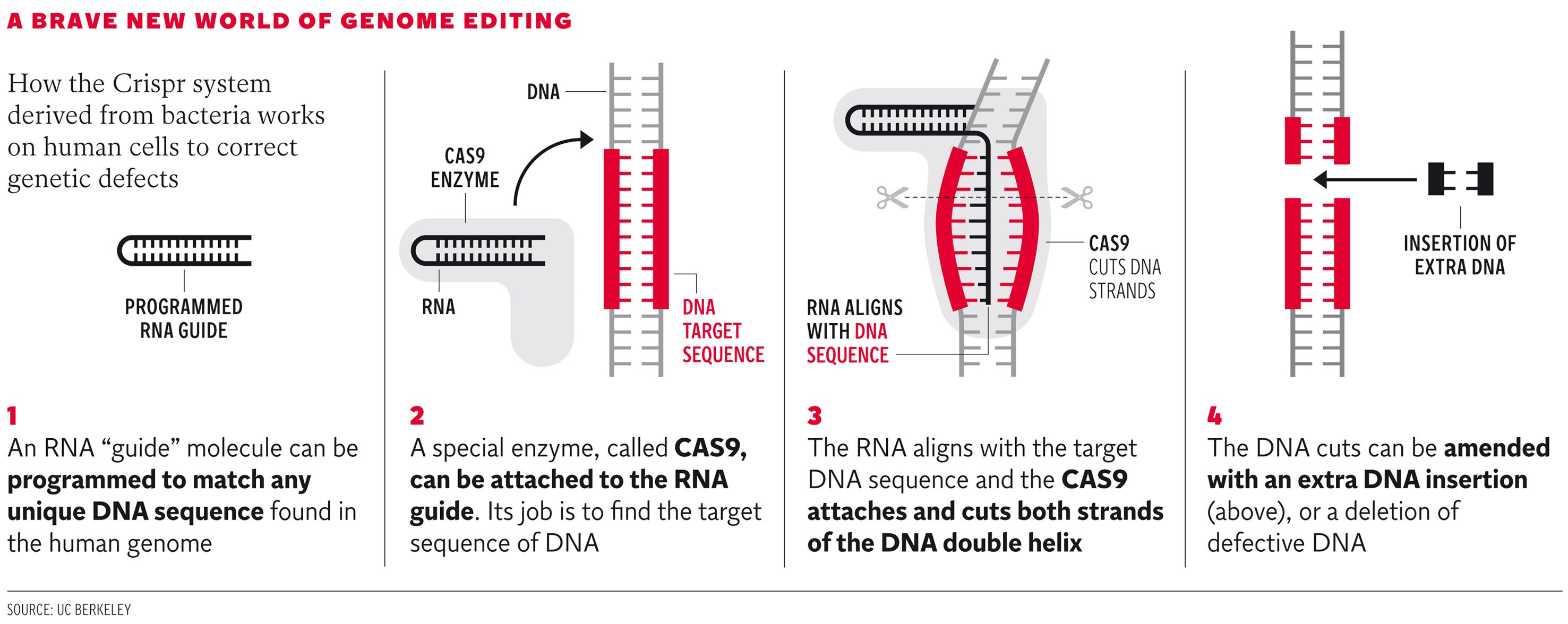
Click image above to enlarge graphic
The development has been hailed as a milestone in medical science because it promises to revolutionise the study and treatment of a range of diseases, from cancer and incurable viruses to inherited genetic disorders such as sickle-cell anaemia and Down syndrome.
For the first time, scientists are able to engineer any part of the human genome with extreme precision using a revolutionary new technique called Crispr, which has been likened to editing the individual letters on any chosen page of an encyclopedia without creating spelling mistakes. The landmark development means it is now possible to make the most accurate and detailed alterations to any specific position on the DNA of the 23 pairs of human chromosomes without introducing unintended mutations or flaws, scientists said.
The technique is so accurate that scientists believe it will soon be used in gene-therapy trials on humans to treat incurable viruses such as HIV or currently untreatable genetic disorders such as Huntington’s disease. It might also be used controversially to correct gene defects in human IVF embryos, scientists said.
Until now, gene therapy has had largely to rely on highly inaccurate methods of editing the genome, often involving modified viruses that insert DNA at random into the genome – considered too risky for many patients.
The new method, however, transforms genetic engineering because it is simple and easy to edit any desired part of the DNA molecule, right down to the individual chemical building-blocks or nucleotides that make up the genetic alphabet, researchers said.
“Crispr is absolutely huge. It’s incredibly powerful and it has many applications, from agriculture to potential gene therapy in humans,” said Craig Mello of the University of Massachusetts Medical School, who shared the 2006 Nobel Prize for medicine for a previous genetic discovery called RNA interference.
“This is really a triumph of basic science and in many ways it’s better than RNA interference. It’s a tremendous breakthrough with huge implications for molecular genetics. It’s a real game-changer,” Professor Mello told The Independent.
“It’s one of those things that you have to see to believe. I read the scientific papers like everyone else but when I saw it working in my own lab, my jaw dropped. A total novice in my lab got it to work,” Professor Mello said.
In addition to engineering the genes of plants and animals, which could accelerate the development of GM crops and livestock, the Crispr technique dramatically “lowers the threshold” for carrying out “germline” gene therapy on human IVF embryos, Professor Mello added.

Germline gene therapy on sperm, eggs or embryos to eliminate inherited diseases alters the DNA of all subsequent generations, but fears over its safety, and the prospect of so-called “designer babies”, has led to it being made illegal in Britain and many other countries.
The new gene-editing technique could address many of the safety concerns because it is so accurate. Some scientists now believe it is only a matter of time before IVF doctors suggest that it could be used to eliminate genetic diseases from affected families by changing an embryo’s DNA before implanting it into the womb.
“If this new technique succeeds in allowing perfectly targeted correction of abnormal genes, eliminating safety concerns, then the exciting prospect is that treatments could be developed and applied to the germline, ridding families and all their descendants of devastating inherited disorders,” said Dagan Wells, an IVF scientist at Oxford University.
“It would be difficult to argue against using it if it can be shown to be as safe, reliable and effective as it appears to be. Who would condemn a child to terrible suffering and perhaps an early death when a therapy exists, capable of repairing the problem?” Dr Wells said.
The Crispr process was first identified as a natural immune defence used by bacteria against invading viruses. Last year, however, scientists led by Jennifer Doudna at the University of California, Berkeley, published a seminal study showing that Crispr can be used to target any region of a genome with extreme precision with the aid of a DNA-cutting enzyme called CAS9.
Since then, several teams of scientists showed that the Crispr-CAS9 system used by Professor Doudna could be adapted to work on a range of life forms, from plants and nematode worms to fruit flies and laboratory mice.
Earlier this year, several teams of scientists demonstrated that it can also be used accurately to engineer the DNA of mouse embryos and even human stem cells grown in culture. Geneticists were astounded by how easy, accurate and effective it is at altering the genetic code of any life form – and they immediately realised the therapeutic potential for medicine.
“The efficiency and ease of use is completely unprecedented. I’m jumping out of my skin with excitement,” said George Church, a geneticist at Harvard University who led one of the teams that used Crispr to edit the human genome for the first time.
“The new technology should permit alterations of serious genetic disorders. This could be done, in principle, at any stage of development from sperm and egg cells and IVF embryos up to the irreversible stages of the disease,” Professor Church said.
David Adams, a DNA scientist at the Wellcome Trust Sanger Institute in Cambridge, said that the technique has the potential to transform the way scientists are able to manipulate the genes of all living organisms, especially patients with inherited diseases, cancer or lifelong HIV infection.
“This is the first time we’ve been able to edit the genome efficiently and precisely and at a scale that means individual patient mutations can be corrected,” Dr Adams said.
“There have been other technologies for editing the genome but they all leave a ‘scar’ behind or foreign DNA in the genome. This leaves no scars behind and you can change the individual nucleotides of DNA – the ‘letters’ of the genetic textbook – without any other unwanted changes,” he said.
Timeline: Landmarks in DNA science
Restriction enzymes: allowed scientists to cut the DNA molecule at or near a recognised genetic sequence. The enzymes work well in microbes but are more difficult to target in the more complex genomes of plants and animals. Their discovery in the 1970s opened the way for the age of genetic engineering, from GM crops to GM animals, and led to the 1978 Nobel Prize for medicine.
Polymerase chain reaction (PCR): a technique developed in 1983 by Kary Mullis (below, credit: Getty) in California allowed scientists to amplify the smallest amounts of DNA – down to a single molecule – to virtually unlimited quantities. It quickly became a standard technique, especially in forensic science, where it is used routinely in analysing the smallest tissue samples left at crime scenes. Many historical crimes have since been solved with the help of the PCR test. Mullis won the Nobel Prize for chemistry in 1993.

RNA interference: scientists working on the changing colour of petunia plants first noticed this phenomenon, which was later shown to involve RNA, a molecular cousin to DNA. In 1998, Craig Mello and Andrew Fire in the US demonstrated the phenomenon on nematode worms, showing that small strands of RNA could be used to turn down the activity of genes, rather like a dimmer switch. They shared the 2006 Nobel Prize for physiology or medicine for the discovery.
Zinc fingers: complex proteins called zinc fingers, first used on mice in 1994, can cut DNA at selected sites in the genome, with the help of enzymes. Another DNA-cutting technique called Talens can do something similar. But both are cumbersome to use and difficult to operate in practice – unlike the Crispr technique.
Click HERE to see a video of how the Crispr system derived from bacteria works on human cells to correct genetic defects
Video by Janet Iwasa
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments