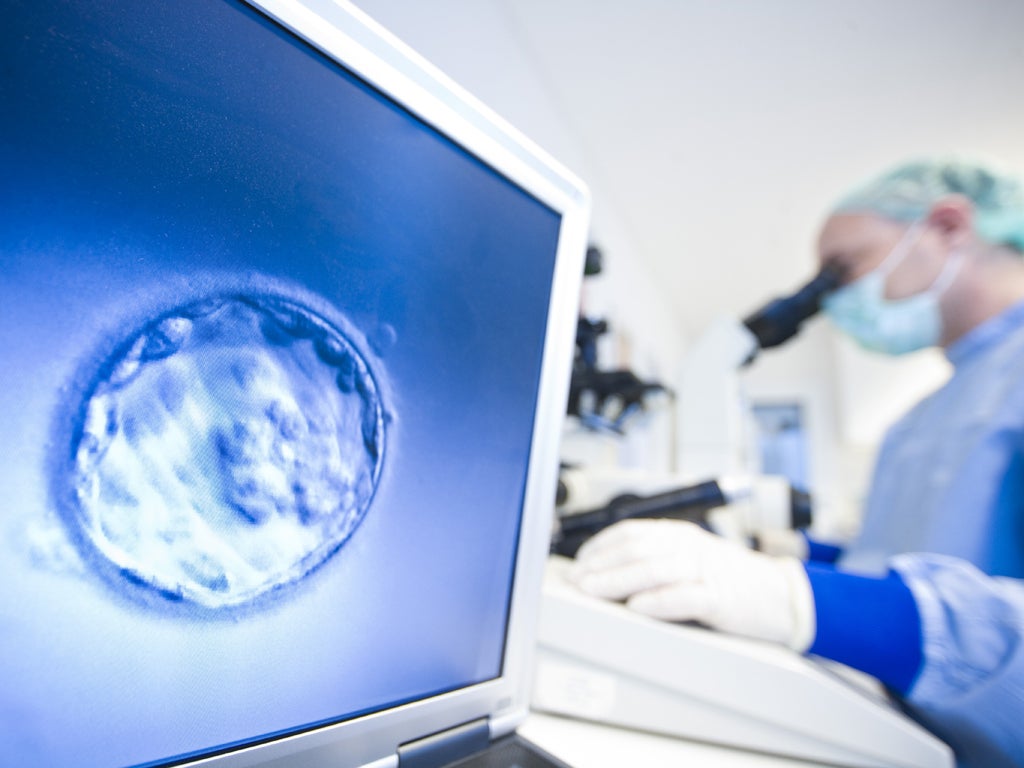
Artificial egg cells used to produce offspring
Experiments using stem-cell technology hailed as 'incredible' fertility breakthrough
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.Scientists have created viable egg cells from skin tissue taken from laboratory mice and have fertilised them in IVF experiments to produce normal, healthy offspring.
The advance is seen as an important breakthrough in attempts to find ways of producing egg cells from infertile women with defective ovaries. It could also lead to the prospect of women having babies after the menopause.
It is the first time that scientists have created viable egg cells and healthy offspring from a mammal using stem cell technology, whereby ordinary cells are converted into stem cells that are then coaxed into becoming mature oocytes, or egg cells, that are fertilised by IVF.
Although the work was carried out on laboratory mice - and there are still huge technical and ethical bridges to cross before it can be applied to humans - scientists are convinced that the breakthrough will one day help infertile women.
The study was carried out by the same team of researchers at Kyoto University in Japan who demonstrated last year that it is possible to generate viable sperm cells in a similar manner by converting skin cells of a mouse into stem cells.
Katasuhiko Hayashi of Kyoto University, who is the first author of the study to be published in the journal Science, said that the egg cells created in the experiment produced healthy mouse offspring which went on to produce their own healthy offspring.
"They grow normally in terms of body weight and size. They have a normal pattern of genomic imprinting [the way genes are inherited] and they are fertile: they generate pups with comparable size of the litter," Dr Hayashi said.
"I must say that it is impossible to adapt immediately this system to human cells, due to a number of not only scientific reasons but also ethical reasons," he said.
The researchers generated the eggs cells using two different methods. The first involved taking stem cells from mouse embryos and culturing them in a broth of chemicals and growth factors to become mature egg cells.
The second method involved genetically engineering the same kind of embryonic skin cells to produce eggs using a technology known as induced pluripotent stem cells (iPSCs).
Evelyn Telfer of Edinburgh University, who was not involved in the study, said the work was a remarkable follow-up from the team's earlier work on producing viable sperms cells from skin cells.
"It is a quite brilliant study. To be able to make an oocyte from scratch as it were really is incredible," Dr Telfer said. However, she added, it will be important to replicate the work using skin cells taken from adult mice rather than mouse embryos, which would be more applicable to humans.
Robert Norman, professor of reproductive medicine at the University of Adelaide in Australia, said that the breakthrough offers hope for infertile women who would like to have their own, genetically-related children.
"[It] offers light to those who want a child who is genetically related to them by using personalised stem cells to create eggs that can produce an offspring that appears to be healthy," Professor Norman said.
"It also offers the potential for women to have their own children well past menopause raising even more ethical issues. Major concerns still need to be address including long-term health of the offspring," he said.
"While this is a major contribution to knowledge, application to humans is still a long way off but for the first time the goal appears to be in sight," he added.
Subscribe to Independent Premium to bookmark this article
Want to bookmark your favourite articles and stories to read or reference later? Start your Independent Premium subscription today.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments