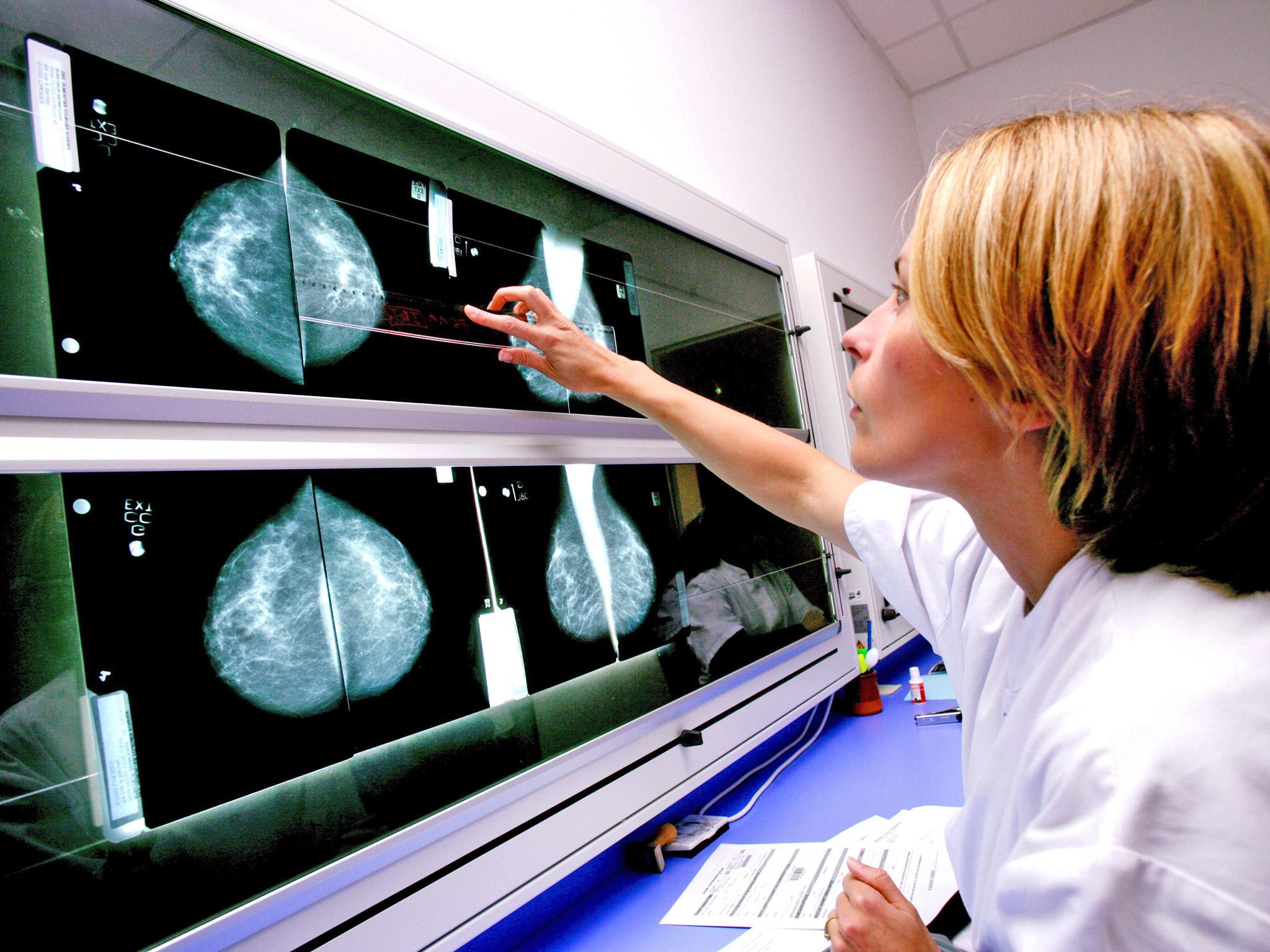
Women with a family history of breast cancer could be offered preventative medication under new NHS plans
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.Around half a million women with a strong family history of breast cancer should be offered drugs to prevent the disease from developing, according to official advice issued today.
The National Institute for Clinical Excellence says in draft guidance that all women over 30 at high risk of breast cancer should be given the opportunity to take drugs to reduce it.
It is the first time that women have been advised to take preventive drug treatment for cancer and represents what one charity called a "historic shift".
Breast cancer is the most common cancer in the UK affecting about 50,000 women and 400 men a year. Most women get the disease by chance and its incidence increases with age.
However, having a family history of breast or ovarian cancer increases the risk. Around 1 per cent of women over 30 are deemed to be at high risk and could be offered tamoxifen or a similar drug raloxifen for five years.
Experts estimate that for every 1000 high risk women who took the drug there would be 20 fewer breast cancers. However, the side effects of the drug, which include an increased risk of blood clots would need to be balanced against the benefits.
Neither drug is yet licensed for preventive treatment . The draft recommendation is out for consultation and final guidance will be published in the summer, NICE said.
Chris Askew, chief executive of the charity Breakthrough Breast Cancer, said: "This draft guideline represents a historic step for the prevention of breast cancer. It is the first time drugs have ever been recommended fro reducing breast cancer in the UK."
The guidance also recommends that high risk women be offered mammography screening tests every year, instead of every three years, and genetic tests to establish if they are carrying a faulty gene, such as BRCA1 or BRCA2, which greatly increases the risk of breast cancer.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments