NHS to give volunteers 'synthetic blood' made in laboratory within two years
It is hoped artificial red blood cells could be used for specialised transfusions
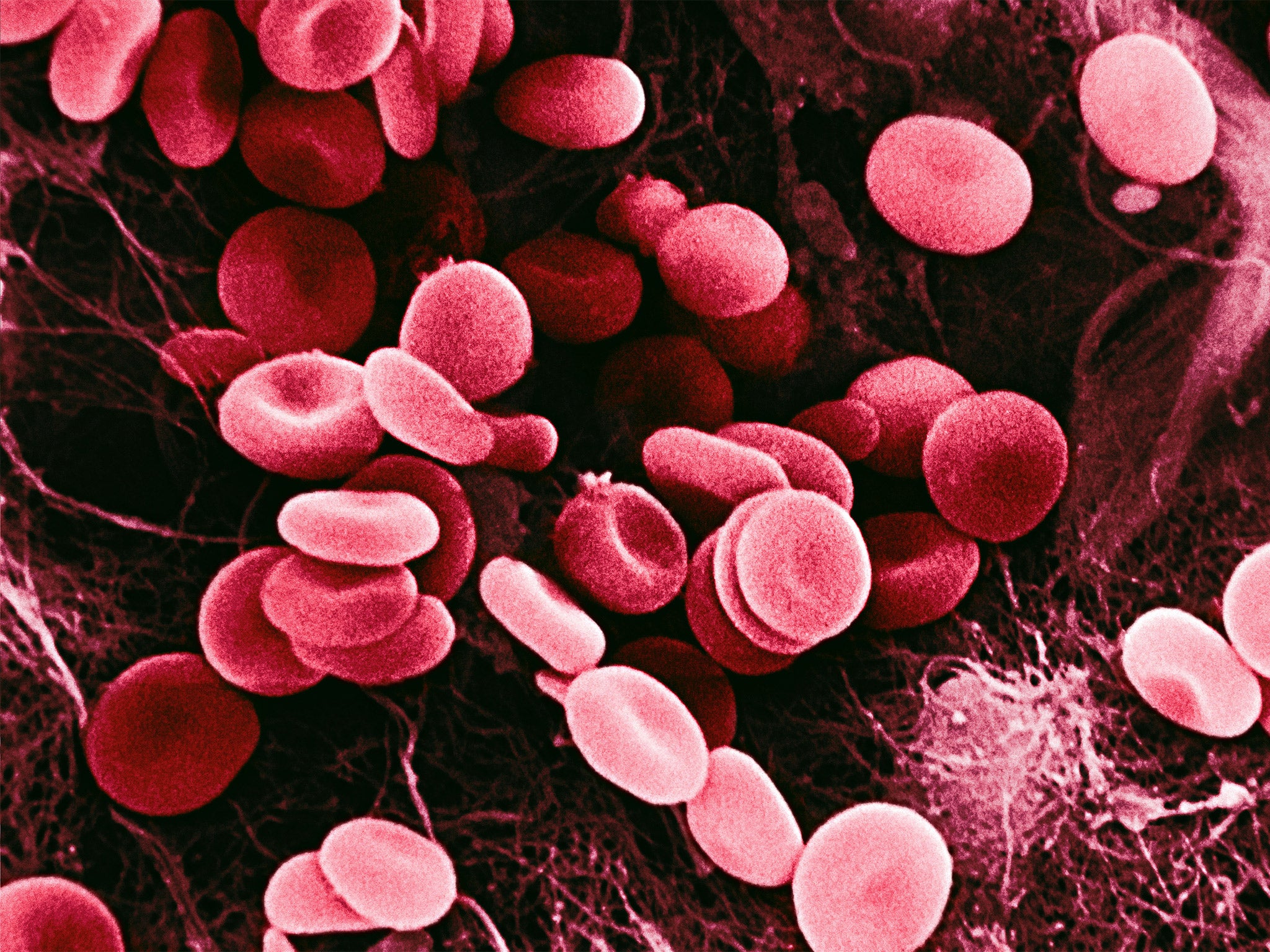
The first attempt at giving human volunteers “synthetic blood” made in a laboratory for the first time will take place within the next two years, the NHS has announced.
A long-awaited clinical trial of artificial red blood cells will occur before 2017, NHS scientists said. The blood is made from stem cells extracted from either the umbilical cord blood of newborn babies or the blood of adult donors.
The trial, thought to be a world first, will involve small transfusions of a few teaspoons of synthetic blood to test for any adverse reactions. It will allow scientists to study the time the manufactured red blood cells can survive within human recipients.
Eventually, it is hoped that the NHS will be able to make unlimited quantities of red blood cells for emergency transfusions. However, the immediate goal is to manufacture specialised donations for patients suffering from blood conditions such as sickle-cell anaemia and thalassemia, who need regular transfusions.
“These trials will compare manufactured cells with donated blood. The intention is not to replace blood donation but to provide specialist treatment for specific patient groups,” said Nick Watkins, assistant director of research and development at NHS Blood and Transplant.
Stem cells isolated from umbilical cord blood donated by pregnant mothers and from the blood of adult donors have been successfully manipulated in the laboratory so they develop into mature, functioning red blood cells, which normally transport oxygen from the lungs to the rest of the body.
The human trials will initially involve transfusions of red blood cells manufactured from the stem cells of adult donors, which if successful will then be followed by transfusions with red cells made from the stem cells extracted from umbilical cord blood donated by consenting mothers, Dr Watkins said.
Tests have shown that these manufactured red cells compare well to the ordinary red blood cells that are made in the body of healthy people. “They are comparable if not identical to the cells from a donor,” Dr Watkins said.
The clinical trials in 2017 are part of a five-year research programme in manufactured red blood cells involving scientists from the universities of Bristol, Cambridge and Oxford. A key aim is to make red cells for patients with complex blood types, where compatible donors are hard to find.
A parallel project is also taking place in Scotland involving the Scottish National Blood Transfusions Service and the Wellcome Trust.
Red blood cells manufactured from stem cells have the advantage of being infection free because the cells have never been inside the human body – which means there is no risk of transmitting viruses such as HIV or hepatitis.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
0Comments