The Independent's journalism is supported by our readers. When you purchase through links on our site, we may earn commission.
Drugs companies putting profit ahead of medical discoveries, warn scientists
Researchers say companies spend vastly more on marketing than on new treatments
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.The multi-billion pound pharmaceutical industry has spent the last decade developing new drugs which have produced little benefit and caused considerable harm, experts say today.
Click HERE to view graphic
The claim that there is an "innovation crisis" in pharmaceuticals because of the difficulty and expense of discovering new drugs is a myth fostered by an industry whose chief focus is on marketing, they add.
Counter to drug industry claims that the pipeline of new drugs is running dry, the number of new drugs being licensed each year has remained at between 15 and 25. But most involve minor tweaks to existing drugs, designed to grab a slice of an existing market rather than offering genuine therapeutic innovation.
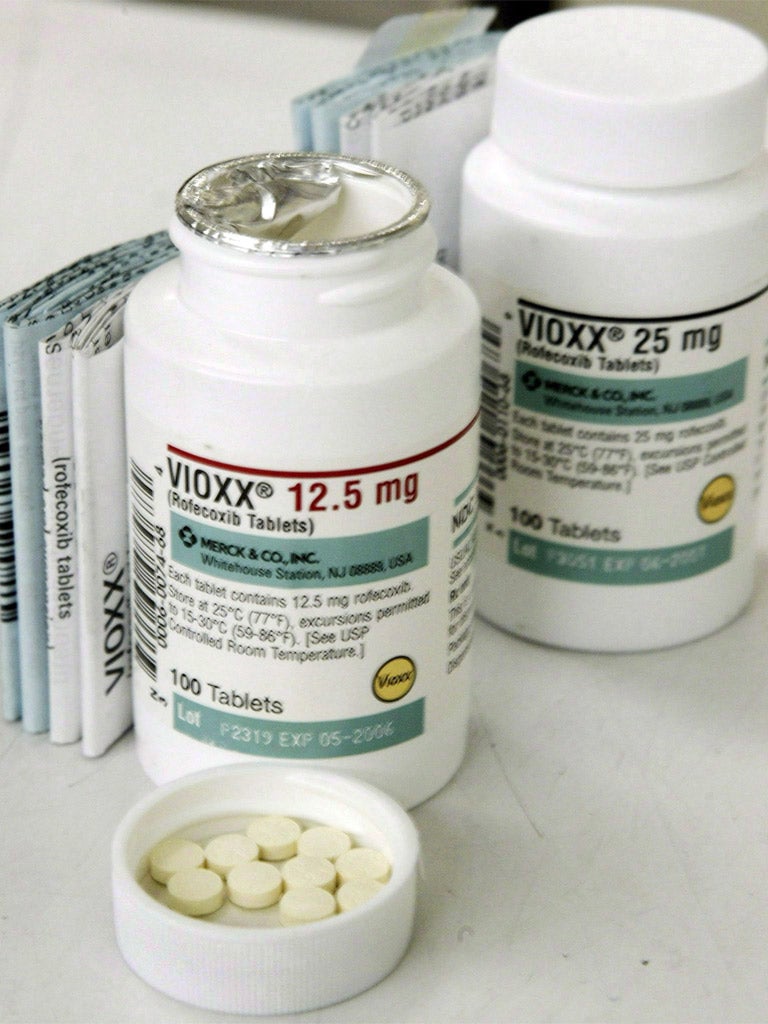
Independent reviews suggest that 85 to 90 per cent provide little benefit over existing treatments with some, such as Vioxx the painkiller and Avandia, the diabetes drug, causing serious side effects which led to their withdrawal, the latter's in Europe.
Writing in the British Medical Journal, Professor Donald Light from the University of Medicine of New Jersey and Joel Lexchin from York University in Toronto say the situation has remained the same for 50 years. The incentives for drug development are wrong and have skewed the behaviour of the industry.
"This is the real innovation crisis: pharmaceutical research and development turns out mostly minor variations on existing drugs and most new drugs are not superior on clinical measures. [They] have also produced an epidemic of serious adverse reactions that have added to national healthcare costs," they say.
More is spent on marketing (25 per cent of revenues) than on discovering new molecules (1.3 per cent). Drug industry claims that the cost of bringing a new drug to market is £1bn and is unsustainable are exaggerated, they claim. Research and development costs did rise substantially between 1995 and 2010 by $34.2bn (£21.9bn), they concluded, but revenues increased six times faster – by $200.4bn.
Companies avoid mentioning this "extraordinary revenue return", they said, adding that up to 80 per cent of drug spending is used by the industry on promotion. The authors call for licensing authorities around the world to stop approving new drugs of little therapeutic value. They suggest large cash prizes should be awarded for genuinely new therapeutic agents in lieu of patent protection.
The European Medicines Agency, which licenses drugs in the UK and Europe, keeps certain data about their safety and efficacy secret. Yet 29 per cent of new biological agents approved by the EMA received safety warnings within the first 10 years.
In a second paper, researchers from the London School of Economics in the UK argue that drug manufacturers should be made to demonstrate that their products are superior to existing treatments before being granted a licence, rather than, as now, superior only to a placebo.
"Changing the nature of regulation could encourage manufacturers to concentrate on the development of new drugs in therapeutic areas with few alternatives," they say. "Supplementing regulation with scientific advice and guidance can steer manufacturer's interest and efforts into key research priorities."
Stephen Whitehead, chief executive of the Association of the British Pharmaceutical Industry, said: "We strongly disagree with the claims made in these papers. Medical research has always rested on iterative and gradual innovation rather than breakthrough advances which are very rare. If it were not for the incremental improvements made in the treatment of HIV, the disease would still be terminal rather than a manageable condition."
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments